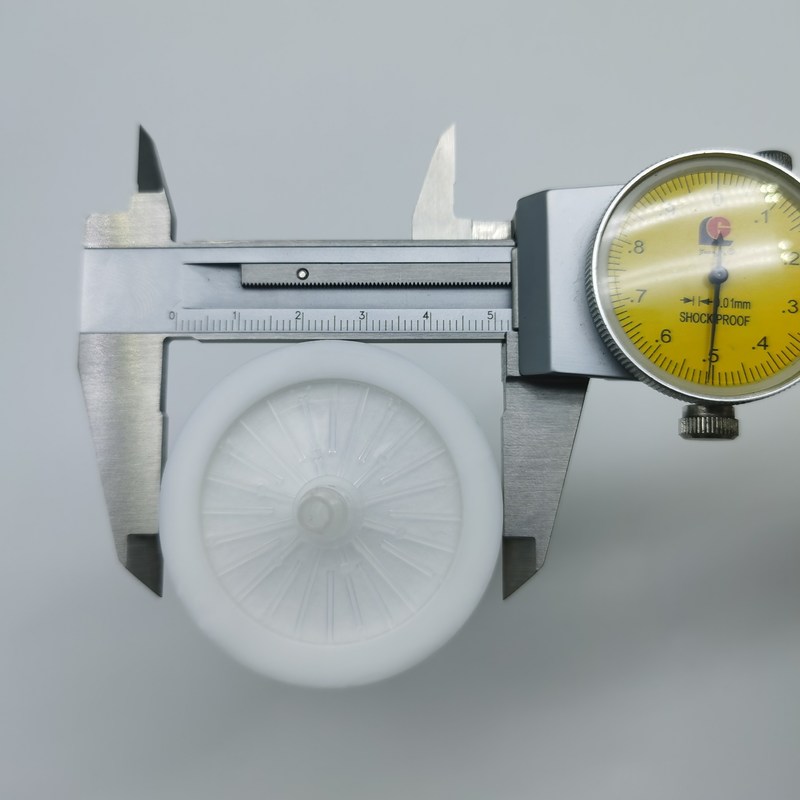
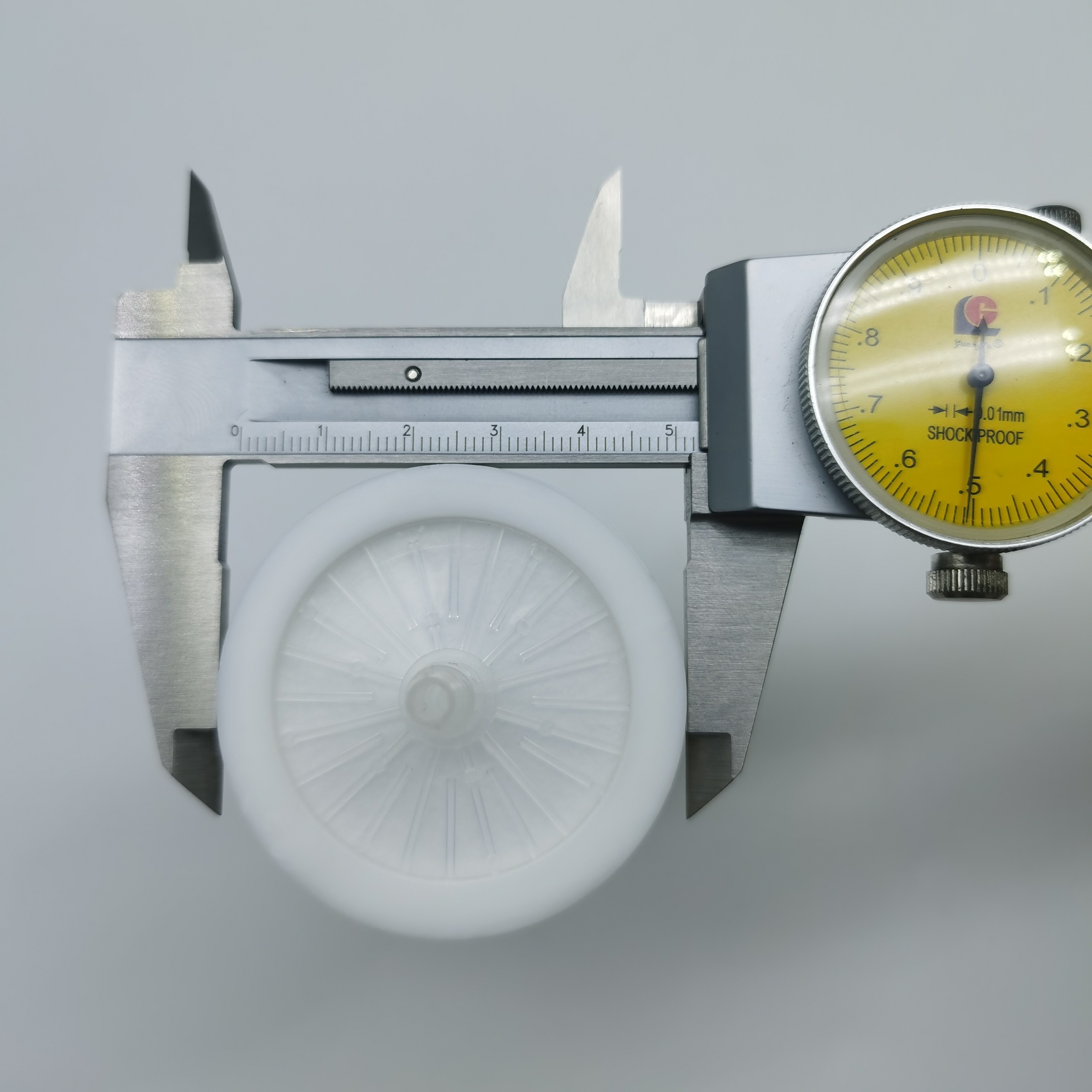
Περιγραφή προϊόντων
Φίλτρο βακτηρίων PTFE 40mm 0,2 micron Υδρόφοβο για Αναρροφητήρα Μονάδας Αναρρόφησης
Το φίλτρο βακτηρίων είναι κατασκευασμένο με μεμβράνη πολυτετραφθοροαιθυλενίου (PTFE), προσφέροντας εξαιρετική αντοχή τόσο στη διάβρωση από οργανικές όσο και από ανόργανες χημικές ουσίες, καθώς και εγγενή υδροφοβικότητα. Χρησιμοποιείται ευρέως στη βιοτεχνολογία, τις φαρμακευτικές βιομηχανίες, τα εργαστήρια και σε κάθε διαδικασία που απαιτεί αποστειρωμένο αερισμό. Το προϊόν είναι ελαφρύ, ασφαλές και αξιόπιστο, χωρίς κίνδυνο συστροφής του σωλήνα που θα μπορούσε να εμποδίσει τον αερισμό.
Χαρακτηριστικά Προϊόντος:
- Φυσικά ισχυρή υδροφοβικότητα, αντοχή στην οξείδωση και αντοχή στη διάβρωση από οργανικές/ανόργανες χημικές ουσίες
- Υψηλός ρυθμός ροής και χαμηλά εκχυλίσιμα
- Ελαφρύς σχεδιασμός για εύκολη εγκατάσταση και αφαίρεση
- 100% δοκιμασμένο για ακεραιότητα
Εφαρμογές:
- Αποστειρωμένος αερισμός δοχείων καλλιέργειας και CO₂ θερμοκοιτίδες
- Αποστειρωμένος αερισμός υγρών μέσων καλλιέργειας
- Αποστειρωμένος αερισμός μικρής κλίμακας ζυμωτήρων
- Αποστειρωμένος αερισμός δεξαμενών αποθήκευσης
- Αερισμός αυτόκλειστου
- Αφαίρεση σωματιδίων αερίου

Προδιαγραφές
Φίλτρο μέσων: Υδρόφοβο PTFE επικαλυμμένο με πολυπροπυλένιο
Διήθηση: 0,22μm
Στέγαση: Πολυπροπυλένιο
Διάμετρος στεγασης: 52mm
Συνδετήρες: 6mm έως 12mm βαθμιδωτό σκληρό
Ύψος στεγασης: 54mm
Διάμετροι φίλτρων μέσων: 40mm
Αποστείρωση: Αυτόκλειστο στους 121℃ 30 λεπτά έως 60 κύκλους
Οι διαδικασίες ανάπτυξης, παραγωγής και πωλήσεων αυτού του προϊόντος συμμορφώνονται με τις απαιτήσεις του Συστήματος Διαχείρισης Ποιότητας ISO 9001.
Οδηγίες Διασφάλισης Ποιότητας
Τα ακόλουθα είναι στοιχεία ρουτίνας επιθεώρησης:
Βιοσυμβατότητα
Τα υλικά αυτού του προϊόντος έχουν δοκιμαστεί και συμμορφώνονται με το USP<88> για τη δοκιμή βιολογικής δραστικότητας VI-121 ℃ πλαστικού.
Καθαριότητα
Αυτό το προϊόν συμμορφώνεται με τον ορισμό ενός «φίλτρου που δεν απελευθερώνει ίνες» όπως ορίζεται στο 21 CFR 210.3(b)(6).
Έμμεσο πρόσθετο τροφίμων
Όλα τα συστατικά υλικά συμμορφώνονται με τις απαιτήσεις του U.S. FDA 21 CFR 177-182 για έμμεσα πρόσθετα τροφίμων.
Όλα τα συστατικά υλικά συμμορφώνονται με τον κανονισμό της ΕΕ 1935/2004/ΕΚ για υλικά που προορίζονται να έρθουν σε επαφή με τρόφιμα.
Μέγιστη Διαφορική Πίεση
Αντέχει σε διαφορική πίεση προς τα εμπρός 3,0 bar στους 20°C.
Μέθοδος Αποστείρωσης
Μετά από 60 κύκλους αποστείρωσης με υγρή θερμότητα (121°C, 30 λεπτά), η δοκιμή ακεραιότητας του φίλτρου παραμένει κατάλληλη.
Κριτήρια Έκδοσης Παρτίδας
Τα φίλτρα που λαμβάνονται από την παρτίδα κατασκευής υποβάλλονται στις ακόλουθες δοκιμές:
Δοκιμή Ακεραιότητας
Κάθε φίλτρο έχει περάσει μια μη καταστρεπτική δοκιμή ακεραιότητας που συσχετίζεται με τη δοκιμή πρόκλησης βακτηρίων.
Σημείο φυσαλίδας ≥ 1,1 bar σε 60% IPA : 40% καθαρισμένο νερό.

 Το μήνυμά σας πρέπει να αποτελείται από 20-3.000 χαρακτήρες!
Το μήνυμά σας πρέπει να αποτελείται από 20-3.000 χαρακτήρες! Παρακαλούμε ελέγξτε το email σας!
Παρακαλούμε ελέγξτε το email σας!  Το μήνυμά σας πρέπει να αποτελείται από 20-3.000 χαρακτήρες!
Το μήνυμά σας πρέπει να αποτελείται από 20-3.000 χαρακτήρες! Παρακαλούμε ελέγξτε το email σας!
Παρακαλούμε ελέγξτε το email σας!